Development of a new assay is most conveniently done using SHC protocol beginning with study of reaction kinetics.
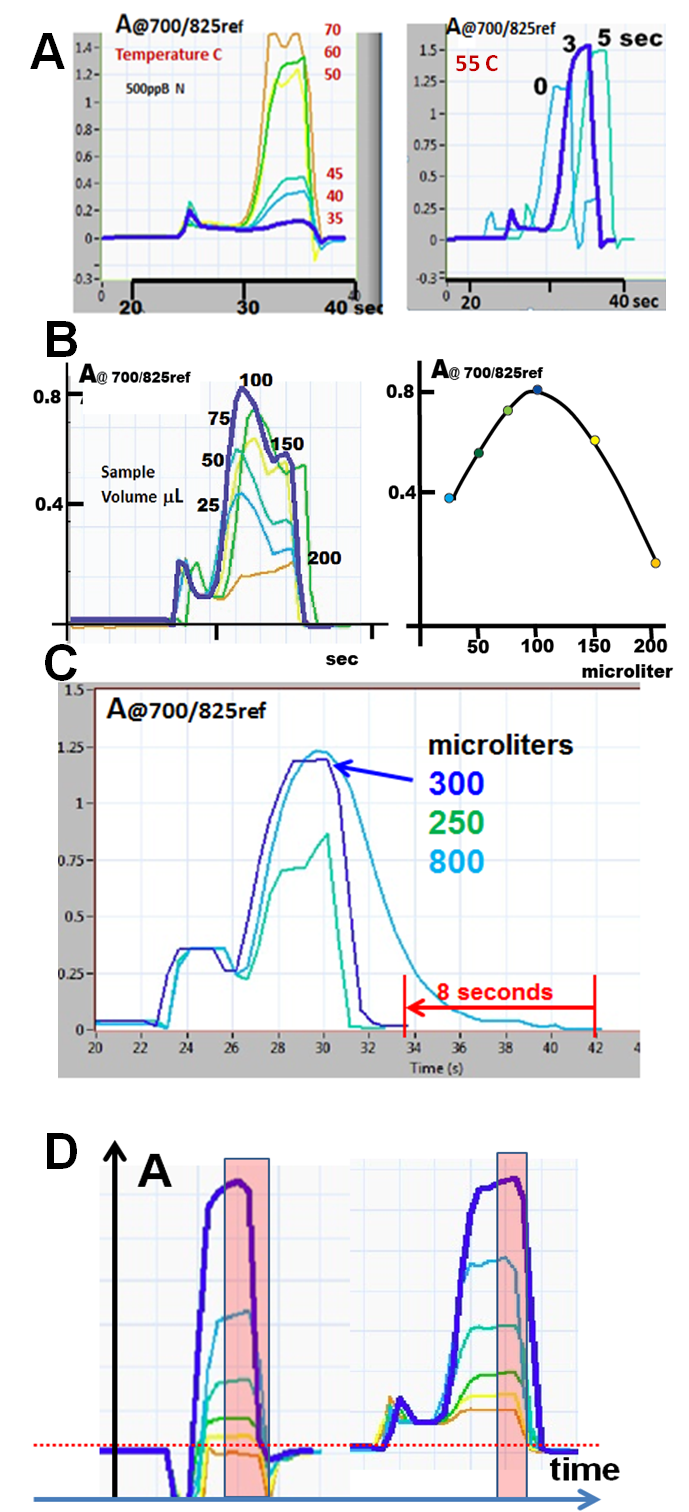
By increasing temperature of the holding coil with a fixed stop period the influence of temperature is investigated first (A left). Since in this case (ammonia assay) a little is gained at higher temperatures, 55 C was chosen to investigate the influence of time by stopping the reaction mixture in the holding coil (A right).
In two reagents, sandwich sequence, increase of sample volume leads initially to increase of response (B), but as the sample volume is further increased, the peak height decreases due to lack of reagent within the sample zone.
During method development, it is practical to monitor flow is sample trough the flow cell at a slow flow rate (50 mcrL/sec), until all reactants are washed out (D blue line). Yet, since in SHC assay the peak height is used to make calibration curve, it is practical to shorten the assay cycle by accelerating the flow right after peak maximum is reached. The volume, allowed to pass the flow cell at a low flow rate, is identified by accelerating the flow rate (to 250mcrL/sec), thus cutting of the trailing edge of the peak. Thus decreasing the slow flow rate volume from 800 mcrL to 250mcrl the optimum cutout volume of at 300 mcrL was identified (D), reducing the sampling cycle from 42 to 33 seconds.
At the interface between carrier (water) and reagents, baseline changes are recorded due to changes of color and refractive index. These changes of absorbance, dependent on reagent composition (D phosphate –left, ammonia-right), are well time resolved. This allows peak height identification trough simple algorithms. At low absorbance levels when changes of baseline are more pronounced (2.2.18.C), advanced data collection programs facilitate this task by defining a time window during which peak maximum is captured.
Tools for Optimization
2.2.24.